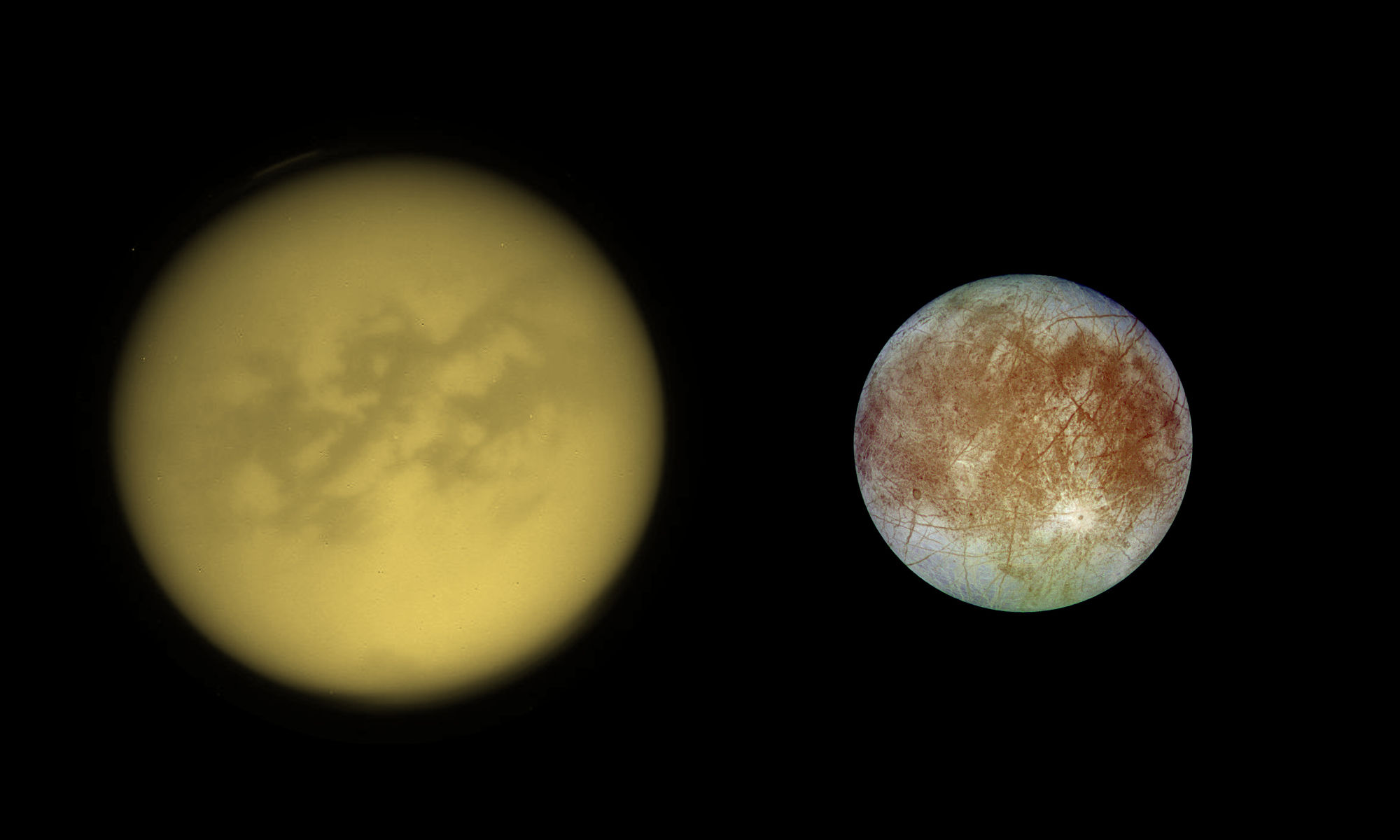
January 23, 2021: Water Can Be Found In The Form Of Clathrate Hydrates On The Surface Of Titan And In The Form Of Ice On The Surface Of Europa According To A New Study
A new study entitled "On the Occurrence of Clathrate Hydrates in Extreme Conditions: Dissociation Pressures and Occupancies at Cryogenic Temperatures with Application to Planetary Systems", published on December 17, 2020 in The Planetary Science Journal and proposed by a team composed of Hideki Tanaka, Takuma Yagasaki and Masakazu Matsumoto reveals that water can form clathrate hydrates on the surface of Titan and can only form ice on the surface of Europa or Ganymede on the basis of theoretical models. The researchers from the Okayama University in Japan developed a statistical mechanics theory in order to predict or to determine the presence of clathrate hydrates on worlds found in the Outer Solar System where environmental temperatures are particularly low. Water is generally found in the form of ice on the surface of the Earth if the environmental temperature is around or below 0 degree Celsius, 32 degrees Fahrenheit or 273 Kelvin. But water can also form clathrate hydrates if the environmental temperature is sufficiently low and if there is the right combination of atmospheric pressure and environmental temperature. The planetologists of the study have been in a position to determine whether Pluto, Titan, Europa or Ganymede can contain clathrate hydrates on their surface.
The presence, the concentration or the amount of clathrate hydrates are likely to bring us major clues regarding the dynamics, the stability or the evolution of any atmosphere for a world where cryogenic temperatures are encountered in the environment. A clathrate hydrate represents a molecular cage which traps small gaseous molecules. Water can appear as a solid, as a liquid or as a gas on our planet but the molecule can also appear in the form of clathrate hydrates on icy bodies located beyond Mars, in the Outer Solar System. In fact, clathrate hydrates represent a particular type of crystalline solid which looks like the typical water ice we know on Earth. Water molecules are widespread in the Solar System. They can be present on the surface of comets, planets, Dwarf Planets or moons. The small water-based cages contain smaller molecules which allow the structure to keep its shape over time or which prevent the molecular cage from collapsing and from generating the typical water ice we regularly encounter on Earth. The key factors for the formation or for the development of clathrate hydrates are pressure and temperature. Numerous moons of the Outer Solar System are devoid of any atmosphere so that water will tend to form the typical ice we know rather than clathrate hydrates.
It takes a relatively long time for clathrate hydrates to form or develop if there are the right environmental conditions so that experimental studies are not obvious to lead rigorously. Planetologists are often surprised to see the contrast between Titan and Ganymede because Titan has a deep and thick atmosphere whereas Ganymede is devoid of any significant atmosphere. The largest moon of Jupiter is in fact bigger and more massive than the largest moon of Saturn and curiously Ganymede has a relatively limited amount of volatiles evolving above it. The contrast is really surprising but the fact that Titan evolves in an area where the level of energy received from the Sun is lower than at the level of Ganymede favors the development of an atmosphere if the world considered is sufficiently massive. Water is expected to be present in the form of ice on the surface of Ganymede and in the form of clathrate hydrates on the surface of Titan. The atmosphere of Saturn's largest moon is dominated by molecular nitrogen and is relatively rich in methane so that methane molecules can be trapped inside potential clathrate hydrates on the surface or within the crust.
The research work performed by the team of Professor Hideki Tanaka, the lead scientist, mobilized both theory and experimental data in order to predict the formation and the development of clathrate hydrates over long periods of time or over geological timescales. Clathrate hydrates can contain various volatiles inside the crystal. On the Opaque Moon Titan, methane which can be present as a liquid on the surface or as a gas in the atmosphere can be trapped inside the molecular cage. Methane molecules trapped in the clathrate hydrates can be slowly released over geological timescales and can fuel the dense atmosphere. The origin of the methane found on Titan is a major subject of research because methane molecules tend to disappear over a period of about 10 million years. Are there internal sources to the methane found in the Titanian atmosphere ? Are there special chemical reactions involving various hydrocarbons or organics ? Hideki Tanaka pointed out: "For many years, we have been developing rigorous statistical mechanics theory to estimate and predict the behavior of clathrate hydrates. In this particular study, we focused on extending this theory to the cryogenic temperature range - down to the 0 K limit."
A major challenge for the group of researchers was to determine the theoretical conditions for the formation and the dissociation of clathrate hydrates under thermodynamic equilibrium at cryogenic temperatures. That phase was fundamental in the prospect of using the famous model of water/hydrate/guest coexistence in clathrate hydrates proposed by van der Waals and Platteeuw in 1959. Hideki Tanaka, Takuma Yagasaki and Masakazu Matsumoto adapted that theory or model to the cryogenic environments encountered in the Outer Solar System to make it applicable on the basis of the thermodynamic data captured by the various space probes like New Horizons, Cassini-Huygens, Voyager 1 and Voyager 2. Titan is the only world in the Outer Solar System where a lander has been sent to the surface. On January 14, 2005, the Huygens probe landed at a low latitude and acquired several aerial views of the landscape as well as a remarkable color view of the surface from the ground. Eroded stones or pebbles could be clearly observed on the surface. Thus, the probe may have landed onto an ancient brook or river. A relatively significant release of methane was identified from the probe during the touchdown.
The surface or the soil of Titan is apparently relatively rich in water ice and methane. The aerial views from the Huygens probe clearly showed the remarkable contrast between bright hills containing a network of dark drainage channels and a dark or brown plain. The new research work tends to show that the surface of the Opaque Moon may contain clathrate hydrates containing methane. The planetologists mobilized the new theory to analyze the state of water on several worlds located beyond our planet in low-temperature environments. They compared the Dwarf Planet Pluto, the largest moon of the Ringed Planet Saturn Titan and Ganymede and Europa, two major moons of the Gas Giant Jupiter. The model tends to show that Pluto and Titan represent worlds where clathrate hydrates can take shape thanks to the right combination of atmospheric pressure and environmental temperature and tends to show that Europa And Ganymede will be worlds where water exclusively appears as ice on the surface. The contrast between those worlds is remarkable and is intimately linked to the absence of a significant atmosphere on Europa and Ganymede.
One can imagine that all the water found on the surface of Titan will appear in its solid form in the particular form of clathrate hydrates which can trap methane molecules inside the crystalline structure. Even if the atmospheric pressure on the surface of Pluto is particularly low, clathrate hydrates may potentially be encountered on the surface as well. Regarding the contrast between the worlds of the Outer Solar System studied here, Hideki Tanaka pointed out: "It is remarkable that one specific state of water appears exclusively in different satellite and planetary surfaces depending on temperature and pressure. In particular, the water in Titan seems to be completely in the form of methane-containing clathrate hydrates all the way up to the surface from the top of its subsurface ocean." Thanks to the Cassini spacecraft, researchers have been in a position to determine that Titan may contain a subsurface layer of liquids beneath the external crust. The nature of the hypothetical ocean is a mystery but that liquid layer may be dominated by liquid water, liquid ammonia or even liquid methane. Cryovolcanic events are likely to fuel the atmosphere with methane, molecular nitrogen or even water ice.
The work on clathrate hydrates in the low-temperature environments of the bodies evolving in the Outer Solar System is likely to tell us a lot regarding the dynamics or the evolution of the atmosphere of worlds like Titan, Triton or Pluto. Water can take surprising forms that we don't regularly encounter on the Blue Planet. Water can be found in its liquid form beneath the icy crust of worlds like Enceladus or Tethys. Water can form the typical ice we know on the surface of most icy worlds devoid of any significant atmosphere. Water can also form clathrate hydrates rather than ice if the atmospheric pressure is sufficiently high and if the environmental temperature is sufficiently low. Saturn's largest moon Titan, whose progressive outward movement from Saturn may influence the axial tilt of the Ringed Planet Saturn according to a recent theory, has unveiled numerous surprises such as the presence of methane or ethane lakes, seas and rivers. Titan looks like the Earth to a certain extent but its meteorology is based on methane rather than water. The interactions between methane and water molecules appear particularly interesting on such a world and can lead to the formation of clathrate hydrates for instance.
- To get further information on that news, go to: http://www.okayama-u.ac.jp/eng/research_highlights/index_id122.html and https://iopscience.iop.org/article/10.3847/PSJ/abc3c0 .