July 17, 2018 : The Ability Of A Type Of Bacteria To Survive In Salty Antifreeze Fuels The Hypotheses Regarding The Potential For Life Beneath The Crust Of Mars, Europa Or Titan
A new study entitled « Enhanced Microbial Survivability in Subzero Brines », published online in April 2018 in Astrobiology and proposed by a team led by Jacob Heinz of the Technical University of Berlin's Center of Astronomy and Astrophysics reveals that bacteria are likely to survive in briny compounds that are present on the Red Planet Mars, on the icy moons Enceladus or Europa, on the Dwarf Planet Pluto or even beneath the crust of Saturn's largest moon Titan. We know that there may be a subsurface ocean on Europa, that there are organic materials on Mars and that there are geysers on Enceladus implying the potential presence of a subsurface ocean with hydrothermal vents. We know that life can adapt to extreme environments on Earth. On our planet, there are bacteria or archaea that can thrive in extreme environments. As a result, is there life elsewhere, in extremely cold environments or in extremely warm environments ? The new work clearly shows the resistance of one type of bacteria. The researchers from the Technical University of Berlin, Tufts University, Imperial College London, and Washington State University studied the behavior of Planococcus halocryophilus, a bacterium that we can encounter in the Arctic permafrost.
We know that there are several worlds in the Solar System that may host a subsurface ocean of liquid water. The temperature of the ocean dominated by water can be below zero degree Celsius if there is a significant concentration of chemicals or salts which generate the phenomenon of antifreeze. Can bacteria or archaea survive or thrive if the concentration of salts is higher or if the environmental temperature is below the usual freezing point of water ? The team of Jacob Heinz wanted to evaluate the survivability of a certain type of microbes. The specialists exposed the type of bacteria to sodium, magnesium and calcium chloride solutions, as well as cocktails of perchlorate. On Mars, perchlorate may allow water to remain liquid on the surface during the Summer season. Jacob Heinz pointed out that his team went well beyond the usual sodium chloride solution because « there's much more than that on Mars. » The group of scientists wanted to determine the level or the concentration of perchlorate from which bacteria didn't survive since we know that perchlorates are toxic in significant concentrations. The researchers observed that the survival rates in perchlorate for that type of microbes were significantly weaker than in all the other solutions but they noticed that at temperatures as low as -30 degrees Celsius or -22 degrees Fahrenheit, the survival rates were a little higher.
Jacob Heinz pointed out that the lowest freezing point depression for perchlorate, which is the lower limit at which the solute reduces the freezing temperature of the solution, is reached if perchlorate represents around 50 percent of the mass of the total solution. That's a figure which is remarkably high compared to that of other chlorides. Researchers are not really surprised by the weak survivability of bacteria in concentrated perchlorate cocktails because perchlorate is known to be particularly toxic. Can life develop if the concentration of perchlorate is relatively high ? That is not unlikely since some extremophiles can adapt to particularly exotic environments. Why not in the harsh environment of Mars where ambient temperatures are very low and where UV light from the Sun can be harmful. Jacob Heinz advanced that the presence of perchlorate « wouldn't preclude life on Mars or elsewhere. » It is in fact a matter of concentration. He pointed out : « Bacteria in ten percent mass perchlorate solutions can still grow. » The soil of the Red Planet hosts less than one weight percent of perchlorate. However, Jacob Heinz insists on the fact that the concentrations of salt in the solutions are different from those in a typical sample of the soil of Mars.
Many factors must be taken into account to evaluate the potential for life on other worlds. Researchers are aware of that. The ability of bacteria to survive depends, for instance, on the level of dilution of liquid perchlorate solutions. If the level of dilution is increased, the survival rate of bacteria will tend to increase. One has to find the right combination between concentration and temperature in order to maximize the survival rate. Theresa Fisher who is a PhD student at Arizona State University's School of Earth and Space Exploration and who is particularly interested in microbial ecology and planetary habitability pointed out that the outcome of the tests doesn't imply that bacteria can't survive on the Red Planet. There are so many types of microbes which can thrive in various environments that we can't rule out the existence of extremophiles that could adapt and thrive in the Martian environment. Planetologists such as Theresa Fisher are aware that the Atacama Desert in Chile which is the driest environment on Earth as well as parts of Antarctica have relatively high concentrations of perchlorate. And we know that microbes or extremophiles evolve in those extreme environments. Theresa Fisher argued : « I'd be surprised if microbes haven't evolved a way to deal with that toxicity. »
As a rule, the survivability of microbes such as bacteria or archaea is boosted by colder temperatures. However, several factors enter into account for the survivability rate and temperature is not a « one-size-fits-all » factor. The survivability rate will also depend on the category of microbe and on the composition of the chemical cocktail. The scientists determined that bacteria in a sodium chloride (NaCl) environment died within two weeks at room temperature. However, at four degrees Celsius, the survival rate rose and once temperatures dropped to as low as -15 degrees Celsius or 5 degrees Fahrenheit, nearly all the organisms survived. Sodium chloride that is to say NaCl which is the usual salt for cooking has a higher freezing point than the other salts since its freezing point is at -21 degrees Celsius or -5.8 degrees Fahrenheit. Researchers noticed that the survival rate of bacteria was significant in the magnesium-chloride (MgCl2) and calcium-chloride (CaCl2) solutions at the particularly low temperature of -30 degrees Celsius or -22 degrees Fahrenheit. Jacob Heinz explained that it is not amazing because « all reactions, including those that kill cells, are slower at lower temperatures, » He continued : « but bacterial survivability didn't increase much at lower temperatures in the perchlorate solution, whereas lower temperatures in calcium chloride solutions yielded a marked increase in survivability. » Therefore, cocktails of perchlorate seem to be more noxious for life than the other salts.
The tests clearly showed that there were differences in the survival rate of bacteria depending on the usual saline solvents. Thus, the researchers determined that, between 4 and 25 degrees Celsius, the survival rate of bacteria was higher in sodium chloride (NaCl) and magnesium chloride (MgCl2) than in calcium chloride (CaCl2). They also noticed however that the survival rate was boosted for any solvent in a harsher environment that is to say with lower temperatures. In the tests, the team of Jacob Heinz exposed the bacteria to multiple freeze/thaw cycles varying from 25 degrees Celsius or 77 degrees Fahrenheit to -50 degrees Celsius or -58 degrees Fahrenheit. Strong temperature changes can occur on Mars. The temperature differences can be particularly high between day and night and the temperature can significantly vary from one season to another season. Like on Earth, the environmental temperature will tend to be higher at lower latitudes or lower at higher latitudes. Today, the mean temperature on the surface of the Red Planet is around -60 degrees Celsius or -76 degrees Fahrenheit. But in the polar areas, the temperature can be as low as -125 degrees Celsius or -193 degrees Fahrenheit. Can bacteria survive in those extreme environments where temperatures significantly vary ?
We have been in a position to notice that the survival rate of bacteria was higher in saltier cocktails. Theresa Fisher pointed out : « Bacteria, when stressed, have shock responses. They manufacture specific proteins that help them adjust, survive, and cope with detrimental environments. » The scientists noted that the death rate of bacteria went down from 20 percent to 7 percent and that the number of freeze/thaw cycles the organisms could resist went up from 70 to 200 when they added 10 percent sodium chloride. Bacteria produce stabilizing proteins in order to resist or to adapt to harsh environments, Theresa Fisher argued, « but there are only so many shock proteins bacteria can produce. » The tests fuel the hypotheses on the probability of life in extreme environments beyond Earth but Jacob Heinz insists on the fact that there is a major difference between surviving and thriving. Bacteria must be in a position to grow which implies a favorable environment.
Jacob Heinz is currently studying the influence of different concentrations of salts at different temperatures on the growth or on the propagation of bacterial colonies. He advanced : « Survival versus growth is a really important distinction. » He added : « But life still manages to surprise us. Some bacteria can not only survive in low temperatures, but require them to metabolize and thrive. We should try to be unbiased in assuming what's necessary for an organism to thrive, not just survive. » There is a multitude of factors that can influence the development of life and in our biosphere, there are extremophiles that can survive or develop in extreme environments. The team of Jacob Heinz explores some limits of life by plunging bacteria into extreme environments with various salt cocktails (toxic perchlorate, NaCl...), with different concentrations of salt and with different temperatures. Can bacteria thrive on Mars, Europa, Enceladus or Titan ? Other factors must be studied as well such as the level of radiations received by bacteria, a high level of atmospheric pressure or a high environmental temperature. The tests upon the limits of life allow us to fuel new hypotheses on the potential for life on other worlds in the Solar System or beyond.
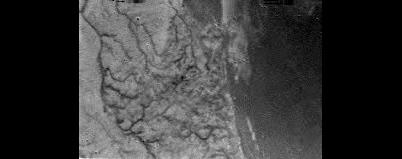
The image above reveals a panoramic view of Titan's landscape from the Huygens probe during its atmospheric descent on January 14, 2005. The raw image was obtained with the Descent Imager/Spectral Radiometer of NASA at an altitude of 16.2 kilometers. One can notice in particular sinuous channels which may be drainage channels. There may be a subsurface ocean of liquid water beneath the crust of the giant moon. Can there be bacteria, archaea or organisms thriving in the extreme environment of the presumed layer of liquid water ? Can life develop in an extremely salty water, in the absence of solar radiations or in a highly pressurized environment ? Image Credit: ESA/NASA/University of Arizona.
- To get further information on that news, go to: https://www.astrobio.net/extreme-life/bacterial-survival-in-salty-antifreeze-raises-hope-for-life-on-mars-and-icy-moons and https://www.liebertpub.com/doi/full/10.1089/ast.2017.1805.